The world in general and the military in particular need a quick, safe, and effective acute treatment for suicidal thinking or ideation (SI). There are good acute medically-based treatments for stroke, heart attacks, and even seizures. However, with SI, there is not a fast-acting effective intervention and thus many patients do not seek treatment when they should. For those who do seek treatment and are admitted to a hospital, medications usually take several weeks to work, and not all patients are good candidates for talking therapy – called cognitive behavioral therapy (CBT). Electroconvulsive therapy (ECT) is overly invasive, requiring general anesthesia, and has cognitive side effects.
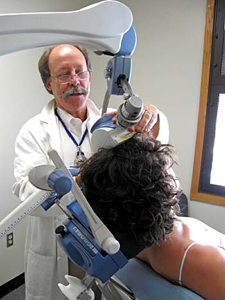
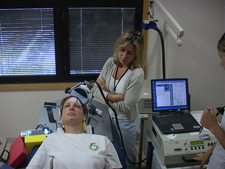
There has been a recent breakthrough with a new treatment called daily left prefrontal transcranial magnetic stimulation (TMS). This non-invasive method was recently (2008) FDA approved for treating acute depression. There are now over 100 machines in psychiatrist’s offices in the US, and a unit at Walter Reed Hospital in Washington. TMS is proving to be a good, non-invasive, non-medication alternative treatment for depression.
Recent studies suggest that higher doses of TMS than were used in the large studies can quickly reduce suicidal thinking, within several days. If this is true, this treatment could transform how we deal with suicidal patients, and save lives. TMS is safe and largely side-effect free. With prior government funding, our group even created a small, portable TMS device able to be used in outpatient clinics and even triage areas or field hospitals. There is intense interest in using this in the VA or DOD, but many unanswered questions remain before it could be widely adopted for treating SI.
This grant is designed to refine how we might use TMS to treat suicidal ideation. It is a clinical trial at two sites – the Ralph H. Johnson VA Medical Center (RHJVAMC) in Charleston, SC, and Walter Reed Army Hospital in Washington DC.
We will enroll 60 suicidal adults admitted over 2 years to either Walter Reed or RHJVAMC. We will give patients TMS or sham TMS for three days on top of their normal treatment. We want to find out whether TMS is feasible in this setting, and whether it reduces suicidal thinking immediately. Even more important, we will follow these patients and look at how long they stay in the hospital, and how their suicidal thinking does for the next 6 months.
This study might help us design and develop a new treatment for suicidal thinking, and eventually be used clinically for soldiers, veterans or their families. If results are positive, because it is non-invasive, TMS could be widely adopted within the DOD to prevent suicides.
In this study, we are testing whether a telephone-based counseling intervention called problem-solving treatment or a control intervention of education alone help service members and National Guard/Reservists with concussion who have returned from deployment to improve symptom control and lessen depression and anxiety. This is a study which is funded by the Department of Defense. We have recruited service members to participate in this study from Madigan Army Medical Center at Joint Base Lewis-McChord and Womack Army Medical Center at Fort Bragg. Three hundred fifty-six service members were enrolled and randomized into one of two groups to receive either telephone counseling calls or educational materials on coping with symptoms and stresses every other week for 6 months.
Problem solving treatment has been used to help treat many types of disorders including depression. By using a telephone to deliver this treatment, the service member’s anonymity will be enhanced (decreasing potential stigma associated with treatment) and the counseling can continue even if the service member is transferred to another base or is discharged from the service.
Data collection ended in December 2014. Staff are currently active checking data, performing data analyses, and generating manuscripts related to the study.